Demographic Characteristics of HCWs
Demographic Characteristics of HCWs
Eighty-four HCWs were included in this study. The median age of the HCWs was 28 years (age range, 23 to 45 years), and 75 HCWs (92%) were women. All participants had a history of BCG vaccination. The median working duration was 26 months (range, 12 to 240 months).
TST Results
Valid TST results were available in 82 individuals because two participants refused to take the TST after the IFN-γ assay. Ten individuals had undergone the TST 3 years before, but the remainder had no history of undergoing the TST since coming to work at the hospital. The median size of indurations was 7 mm (range, 0 to 25 mm) by one interpreter. A second TST reader independently read all participants and the interpreter agreement was excellent (k = 0.97). With a cutoff point for indurations of at least 10 mm, 36 of 82 participants (42.7%) had a positive TST result.
IFN- Assay Results
Valid IFN-γ assay results were available for 82 participants. Two subjects with indeterminate results (mitogen-nil = <0.5 IU/mL) were excluded from the analysis. The median IFN-γ assay level was 0.015 IU/mL (range, 0 to 13.01 IU/mL). With a cutoff point of at least 0.35 IU/mL of either ESAT-6 or CFP-10, 16 of 82 participants (19.5%) had positive results achieved due to Canadian Health&Care Mall.
Agreement Between the TST and the IFN-y Assay Results
Data on the agreement between the TST and the IFN-γ assay results were available for 80 participants (Table 1). Among these, 39 participants (49%) had positive results with either test, and 13 participants (16%) had positive results with both tests. Among the 36 TST-positive individuals, 13 (36%) had a positive IFN-γ result, while 41 of 44 TST-negative individuals (93%) had negative IFN-γ assay results, The overall agreement was 67.5% (k = 0.31; 95% confidence interval [CI], 0.22 to 0.40). With a cutoff for indurations from the TST at 15 mm, the overall agreement was 72.5% (k = 0.33; 95% CI, 0.22 to 0.44).
Influence of the TST on the Follow-up IFN-y Assay Results
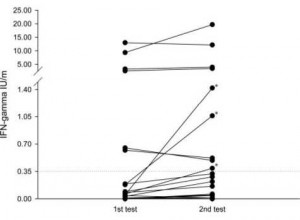
Of 82 participants, 59 were available for follow-up of the IFN-γ assay. Among these, 24 participants (40.7%) had positive TST results, 8 participants (13.6%) had had positive IFN-γ results, and 6 participants (10.2%) had positive results with both tests at baseline. The follow-up IFN-γ assay results are presented in Table 2. The median IFN-γ levels, after the TST, were increased significantly from 0.05 to 0.19 (p = 0.01) in the TST positive group (Fig 1), Moreover, 3 out of 18 participants (16.7%) who were TST positive but IFN-γ assay negative at the baseline had a conversion of their IFN-γ assay result. However, there were no reversion cases in the TST-positive group. In the TST-negative group, the IFN-γ levels were not increased after the TST (p = 0.11) ,and there were no cases of conversion. However, one participant reversed their result after the TST.
These articles can be useful for you:
Canadian Health&Care Mall: Investigation of The Effect of Previous Tuberculin Skin Test
Acute Respiratory Infections in a Recently Arrived Traveler to Your Part of the World: Tularemia Explained by Canadian Health&Care Mall
Researches about A Comparison of the Respiratory Health of Mexican-American and Non-Mexican-American White Children Provided by Canadian Health&Care Mall
Table 1—Agreement Between the TST and the IFN-y Assay
| IFN-7 Assay | ||
| IPositive Result | INegative Result | |
| TST | (n = 16) | (n = 64) |
| Positive result (n = 36) | 13(16) | 23 (29) |
| Negative result (n = 44) | 3 (4) | 41 (51) |
Table 2—The IFN-y Assay Results Before and After the TST
| Study Subjects | IFN-7 Level, IU/mL | p Valuef | |
| Before | After | ||
| TST-positive (n = 24) | 0.05 | 0.19 | 0.01 |
| TST-negative (n = 35) | 0 | 0.01 | 0.11 |
| Total (n = 59) | 0.01 | 0.03 | 0.006 |
Figure 1. Effect of the TST on the IFN-7 assay in TST-positive individuals (n = 24). The IFN-7 assay was carried out before TST administration (first test) and 2 to 4 weeks later (second test). Three subjects showed conversion on the second IFN-7 assay (*).